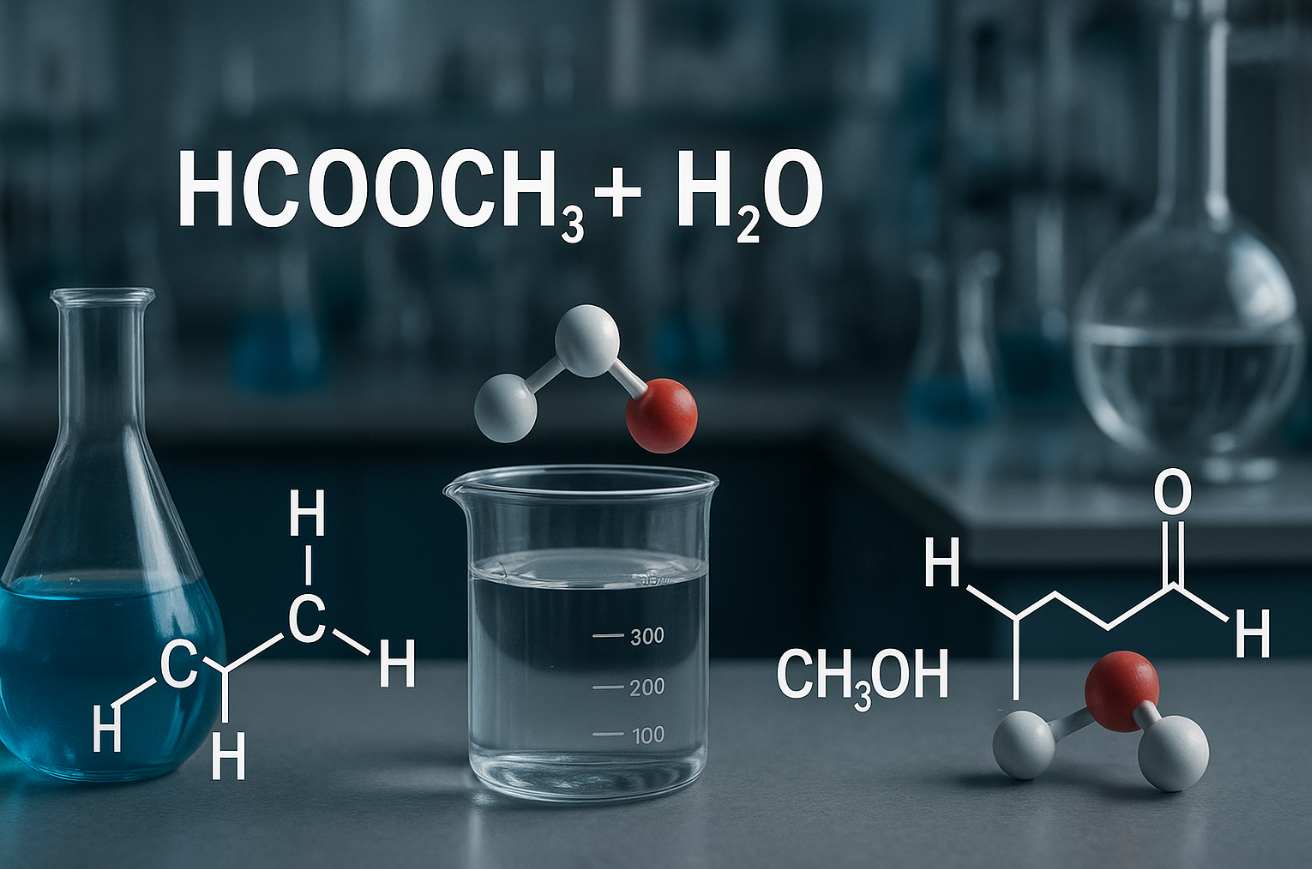
HCOOCH CH2 H2O is more than just a complex chemical formula; it represents a fascinating intersection of chemistry and real-world application. This compound, also known as formic acid methyl ether in aqueous solution, has garnered attention for its unique properties and versatility. From its intriguing molecular structure to its various uses across industries, understanding HCOOCH CH2 H2O can open doors to innovative solutions in science and technology.
In this blog post, we will explore the fundamentals of HCOOCH CH2 H2O, diving into its chemical behavior, reactivity patterns, and practical applications. Whether you’re a student eager to learn or an industry professional seeking insights into new trends, you’ll find valuable information that highlights the importance of this compound in both laboratory settings and industrial environments. Let’s unravel the chemistry behind HCOOCH CH2 H2O together!
Fundamentals of HCOOCH CH2 H2O
HCOOCH CH2 H2O is a chemical compound formed by the combination of formic acid and methanol in an aqueous solution. This unique structure holds significant interest due to its potential as a solvent and reagent in various chemical reactions.
The presence of both functional groups allows for versatile interactions. The formate group contributes to hydrogen bonding capabilities, enhancing solubility in water. Meanwhile, the methyl ether component increases stability under certain conditions.
As researchers delve deeper into its characteristics, they uncover more about its applications across different fields such as agriculture, pharmaceuticals, and materials science. Understanding these fundamentals sets the stage for exploring its practical uses and innovations.
Molecular Structure of HCOOCH CH2 H2O
The molecular formula HCOOCH CH2 H2O represents an intriguing compound with unique characteristics. Its structure consists of a formate group (HCOO) linked to a methylene unit (CH2), indicating the presence of carbon and oxygen in distinct arrangements.
The molecule exhibits polar covalent bonds, which significantly influence its physical properties. Carbon atoms serve as a backbone, while the hydroxyl component adds hydrophilic traits, enhancing solubility in water.
This blend results in a versatile compound utilized across various applications. Understanding its molecular structure provides insight into its reactivity and potential uses in both industrial and laboratory settings.
HCOOCH CH2 H2O: The Chemical Reaction
HCOOCH CH2 H2O, also known as methyl formate, undergoes several key chemical reactions. The esterification process primarily involves the reaction of methanol with formic acid. This condensation reaction results in the formation of methyl formate and water.
In hydrolysis, methyl formate can react with water to produce methanol and formic acid. This reversible reaction is significant in various applications, such as organic synthesis and industrial processes.
Additionally, under specific conditions, HCOOCH CH2 H2O can take part in transesterification reactions. Here, it exchanges its alkoxy group for another alcohol’s alkoxy group, yielding different esters that have diverse functional uses across multiple sectors.
Reactivity Patterns of HCOOCH CH2 H2O
HCOOCH CH2 H2O exhibits notable reactivity patterns due to its molecular structure. Its ester functional group allows for hydrolysis under acidic or basic conditions, leading to the formation of methanol and formic acid. This reaction is essential in organic synthesis.
Further reactions may occur through nucleophilic substitution, where other reactants can replace the -OCH3 group. These transformations are valuable in creating complex molecules in pharmaceutical chemistry.
Additionally, HCOOCH CH2 H2O can participate in condensation reactions with alcohols or amines, generating esters or amides. Such versatility makes it a key compound in synthetic organic chemistry applications across various industries.
Industrial Applications of HCOOCH CH2 H2O
HCOOCH CH2 H2O serves as a crucial intermediate in various industrial processes. It is commonly utilized in the production of specialty chemicals and polymers, enhancing product performance and stability. This compound plays a pivotal role in formulating surfactants that improve wetting properties.
Additionally, its applications extend to pharmaceuticals, where it aids in drug formulation and delivery systems. The ability to modify molecular structures allows for tailored therapeutic effects.
Moreover, HCOOCH CH2 H2O finds utility in agricultural chemistry as well. Its effectiveness as a biodegradable solvent makes it ideal for developing eco-friendly pesticides and herbicides, promoting sustainable farming practices.
Laboratory Techniques with HCOOCH CH2 H2O
Laboratory techniques involving HCOOCH CH2 H2O are essential for various chemical analyses. One standard method is NMR spectroscopy, which helps determine the molecular structure and purity of this compound. By analyzing shifts in resonance frequencies, researchers can glean crucial information about its behavior.
Another technique includes chromatography, particularly gas or liquid chromatography. This allows scientists to separate and quantify components within mixtures containing HCOOCH CH2 H2O. It’s vital for assessing concentrations in samples.
Additionally, titration methods can be employed to gauge reactivity and stability under different conditions. Accurate measurements enable a better understanding of this compound’s properties and potential applications in industrial settings.
Environmental and Safety Considerations for HCOOCH CH2 H2O
HCOOCH CH2 H2O presents both environmental and safety considerations that must be addressed. This compound can pose risks if not handled properly, as it may release volatile organic compounds (VOCs) during synthesis or application.
Proper ventilation in workspaces is crucial to minimize inhalation exposure. Safety protocols should include the use of personal protective equipment like gloves and masks when handling this substance to prevent skin or respiratory irritation.
Disposal methods also require careful planning and following local regulations for hazardous materials. Ensuring responsible management helps mitigate any potential negative impact on the environment while safeguarding human health during its use and disposal.
Emerging Research Trends for HCOOCH CH2 H2O
Emerging research on HCOOCH CH2 H2O is focusing on its potential as a green solvent. Scientists are exploring how this compound can replace traditional solvents in various chemical reactions. The aim is to reduce environmental impact while maintaining efficiency.
Another exciting trend involves studying the compound’s role in catalysis. Researchers are investigating its effectiveness in accelerating chemical processes, which could lead to more sustainable manufacturing practices.
Additionally, ongoing studies examine the interactions of HCOOCH CH2 H2O with biological systems. Understanding these interactions may open new avenues for therapeutic applications and drug development, showcasing the versatility of this intriguing molecule.
Frequently Asked Questions
HCOOCH CH2 H2O raises various questions among researchers and industry professionals. One common inquiry is about its stability under different conditions. Generally, it shows resilience but can react with strong acids.
Another frequent question revolves around its solubility in water and organic solvents. Its unique molecular structure allows for moderate solubility, making it useful for diverse applications.
Many wonder about the potential hazards associated with handling HCOOCH CH2 H2O. While it’s relatively safe when appropriately managed, appropriate safety measures are essential to mitigate any risks during laboratory or industrial use.
Conclusion
The study of HCOOCH CH2 H2O opens up diverse avenues in both research and practical applications. Its unique molecular structure and reactivity patterns highlight its potential across various industries. From chemical synthesis to innovative laboratory techniques, the versatility of this compound cannot be overstated.
As environmental concerns grow, understanding safety protocols becomes crucial for handling HCOOCH CH2 H2O responsibly. Emerging research continues to unveil new possibilities, suggesting that we have only begun to scratch the surface of what this compound can achieve.
Whether you are a researcher or an industry professional, keeping abreast of developments related to HCOOCH CH2 H2O is essential for leveraging its full potential responsibly and effectively.